Registration of medical devices in the EAEU
Registration of medical devices within the Eurasian Economic Union (EAEU) takes place in accordance with the Decision of the Council of the Eurasian Economic Commission dated February 12, 2016 No. 46 «Rules for registration and examination of the safety, quality and effectiveness of medical devices».
Registration of modifications of medical devices
In case of simultaneous submission for registration of several modifications of a medical device belonging to the same type of medical device in accordance with the nomenclature of medical devices used in the Union, manufactured by one manufacturer, differing from each other in changes in the configuration and (or) technical parameters that do not affect the principle of operation and functional appointment belonging to the same class of potential risk of use, the applicant submits 1 application and 1 registration dossier.
If the submitted modifications relate to different types of medical device in accordance with the specified nomenclature, each modification is registered separately with the provision of a separate registration dossier.
If the submitted modifications relate to different types of medical device in accordance with the specified nomenclature, each modification is registered separately with the provision of a separate registration dossier.

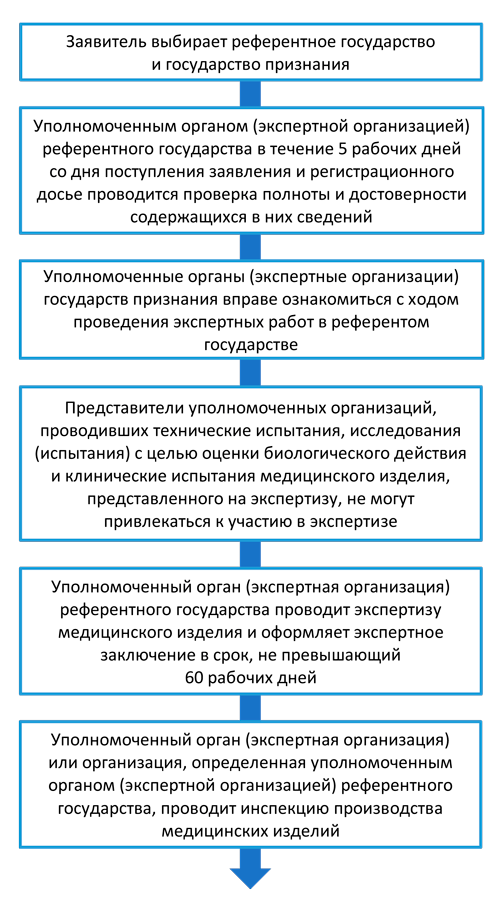
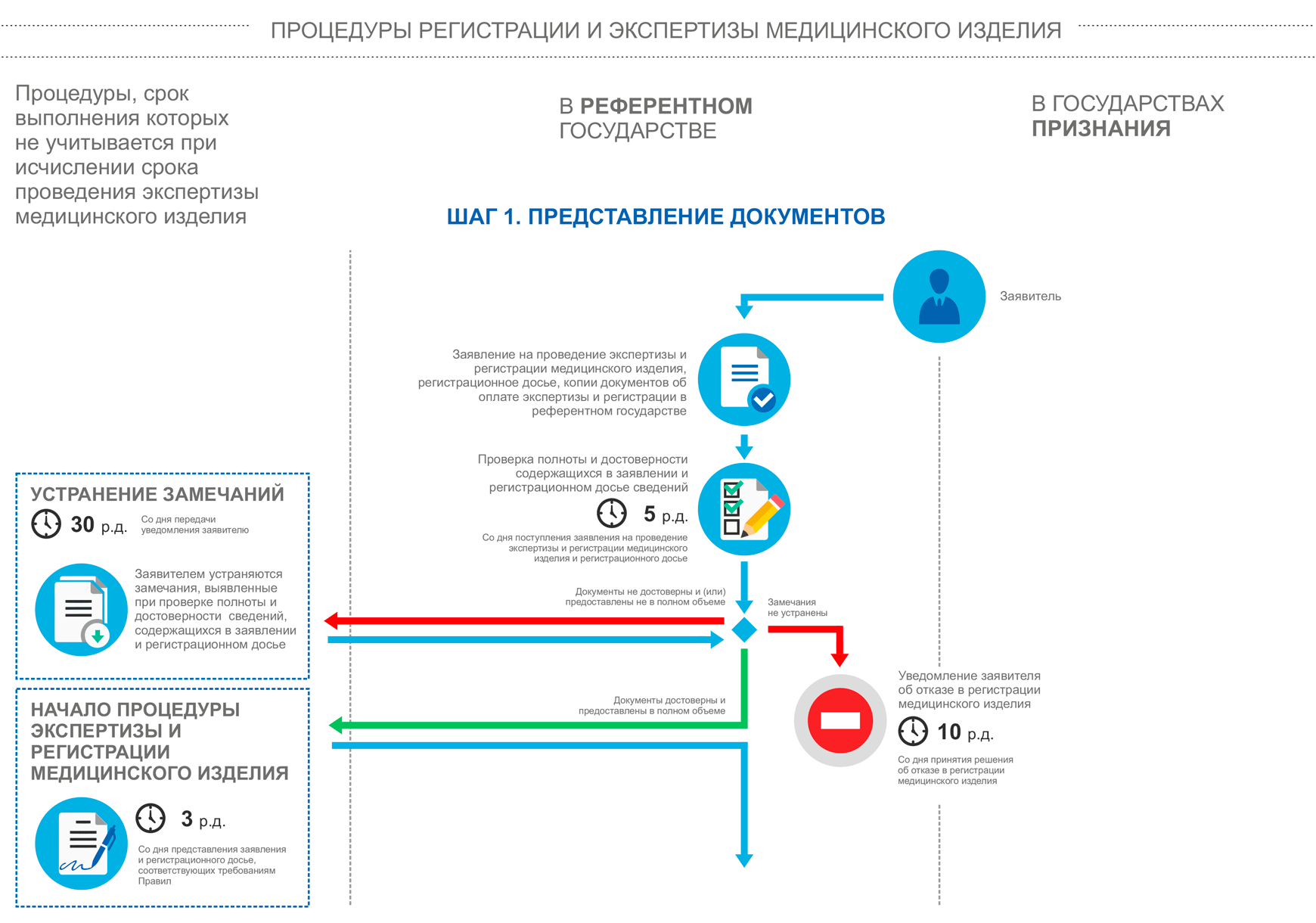
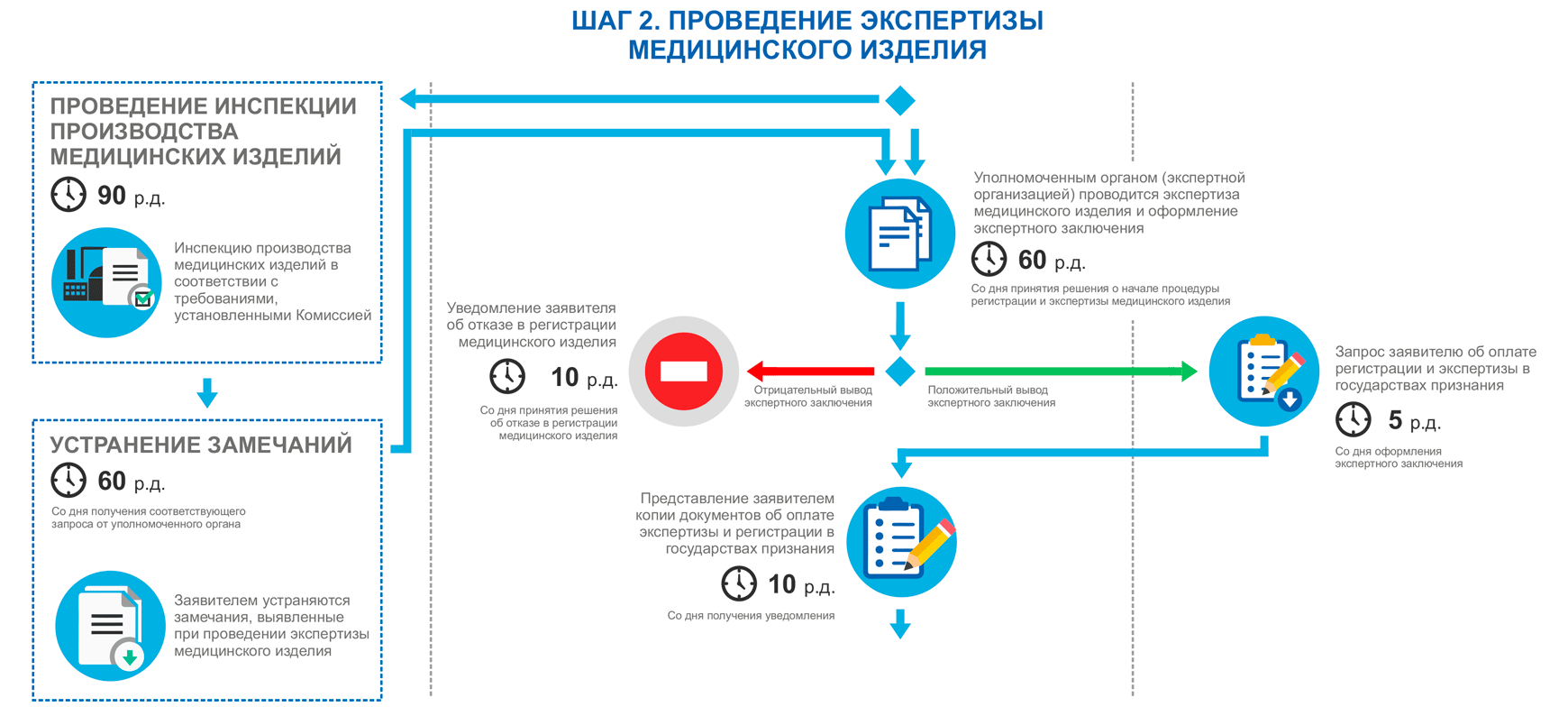
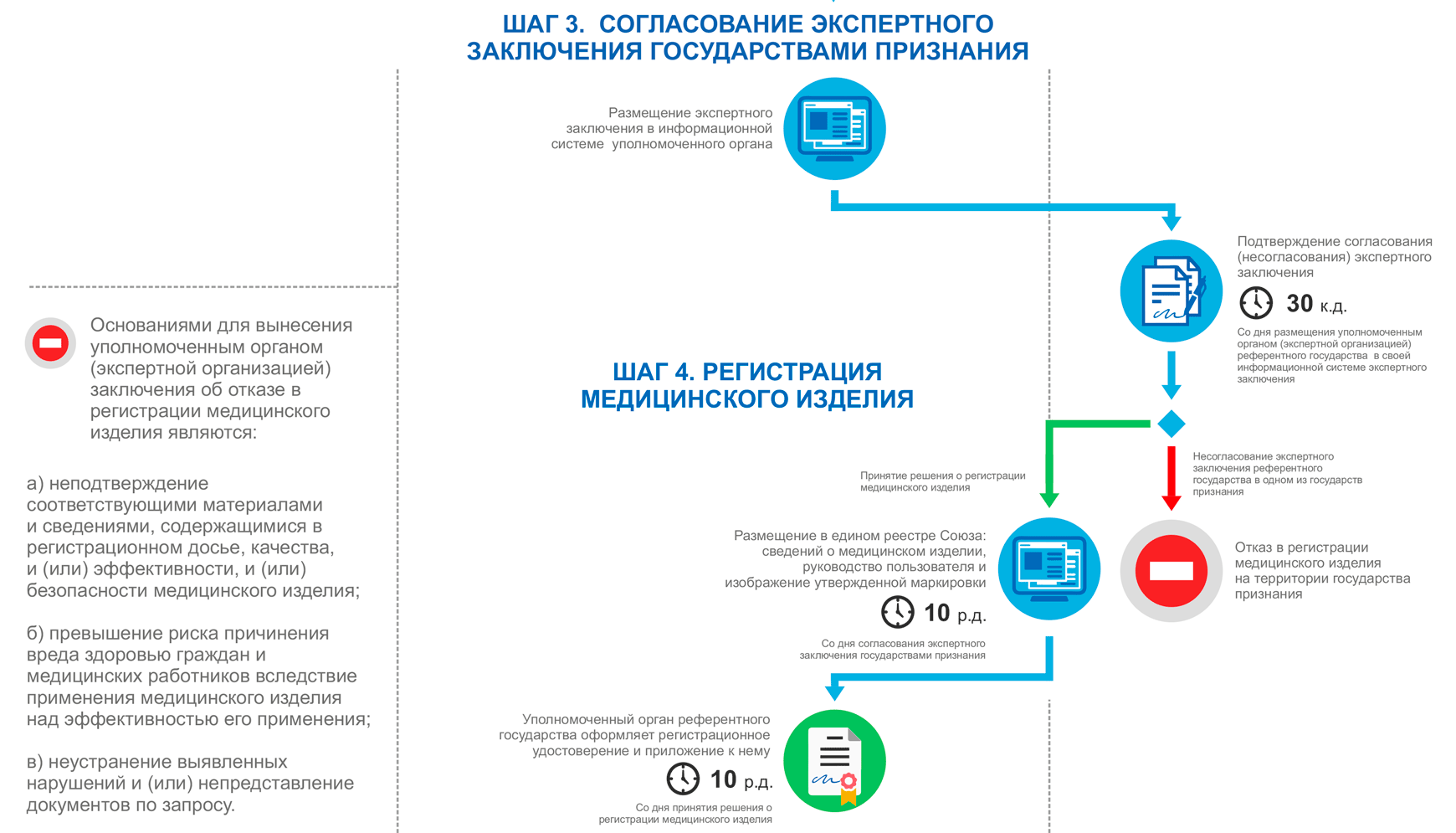
Stages of device registration:

Registration of products in the EAEU, step 2

Registration of products in the EAEU, step 3
The amount of the state duty:
State duty for the issuance of a registration certificate for a medical device of the EAEU – 11 000 rubles
State duty for conducting an examination of the safety, quality and effectiveness of a medical device during its registration:
State duty for conducting an examination of the safety, quality and effectiveness of a medical device during its registration:
- Class 1 – 72 000 rubles;
- Class 2a – 104 000 rubles;
- Class 2b – 136 000 rubles;
- Class 3 – 184 000 rubles.
Cost calculation:
List of information required to calculate the cost of registering a medical device:
- Name of the medical device;
- Purpose and scope;
- Specifications;
- The materials from which the medical device is made;
- Analogues registered in the Russian Federation.
If you are interested in this service, please contact us
SERVICES
Fill out the form for feedback
By clicking on the button, I agree to the processing of personal data.