Registration of medical devices in Russia
Registration of medical devices is a procedure, the purpose of which is to authorize the circulation of medical devices. After passing the registration procedure for medical devices, a document is issued – a Registration Certificate.
Medical devices include:
- Tools;
- Devices;
- Appliances;
- Equipment;
- Materials;
- Other products used for medical purposes alone or in combination with each other and / or other accessories;
- Special software.
Decree of the Government of the Russian Federation of December 27, 2012 No. 1416 defines the Rules for the state registration of medical devices.

Risk classification:
- Class 1 – medical devices with a low degree of risk;
- Class 2a – medical devices with an average degree of risk;
- Class 2b – medical devices with a high degree of risk;
- Class 3 – medical devices with a high degree of risk
* The state registration of medical devices is carried out within a period not exceeding 50 working days from the date of the decision to start the state registration of medical devices;
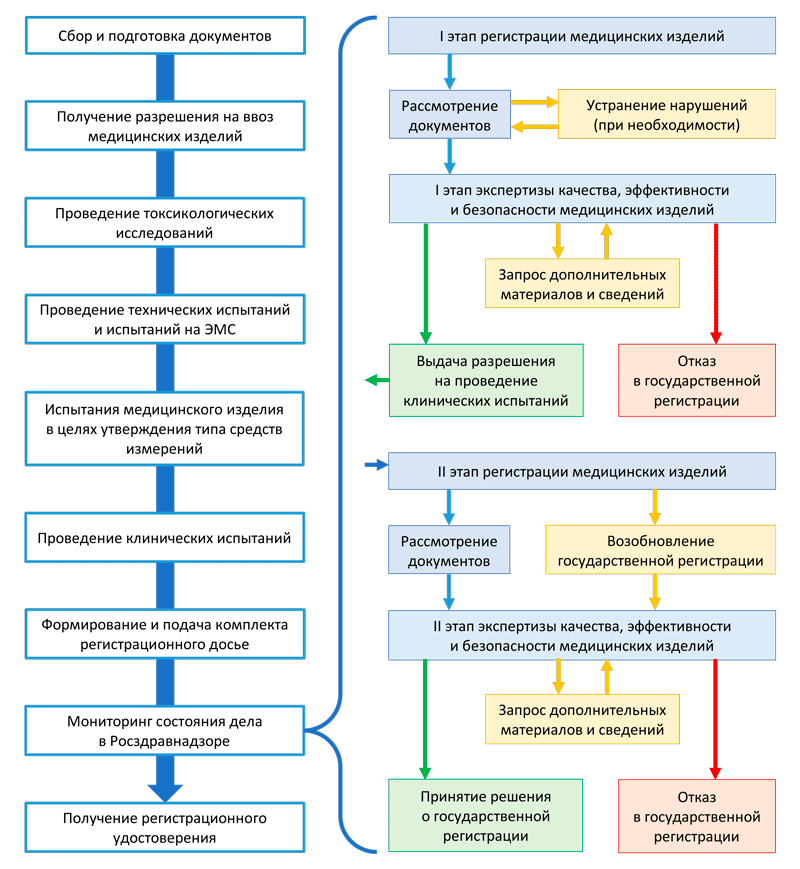
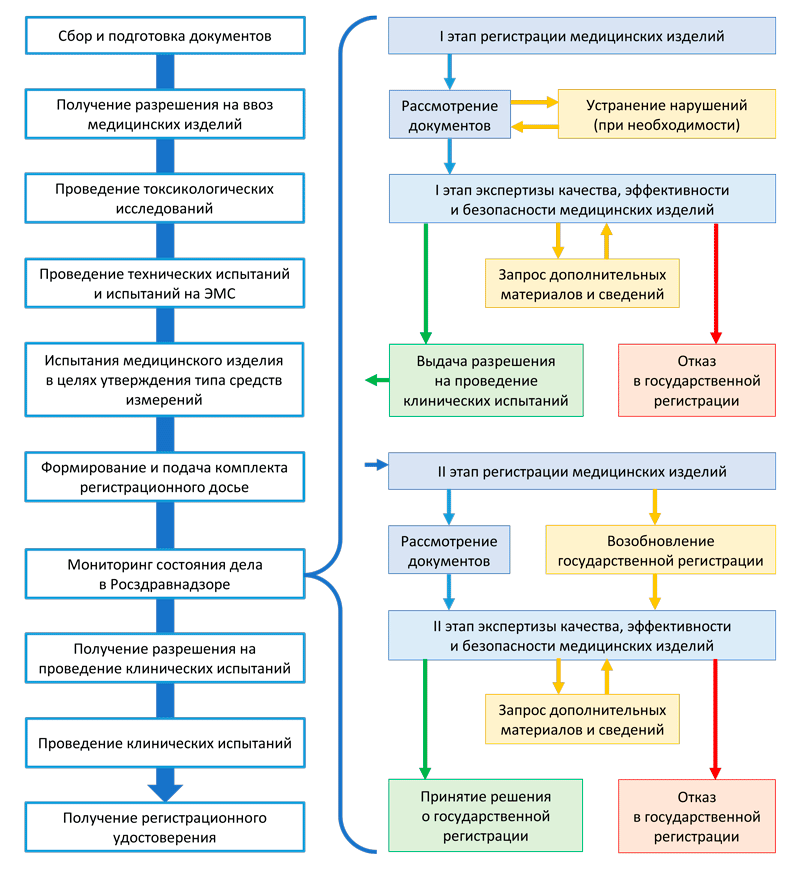
** The term for conducting clinical trials of a medical device of risk class 2a, 2b and 3 is not included in this 50-day period.Depending on the risk class of the medical device, registration takes place in the following stages:
The amount of the state duty:
State duty for registration of a medical device – 11 000 rubles
State duty for the examination of the quality, efficiency and safety of medical devices:
State duty for the examination of the quality, efficiency and safety of medical devices:
- Class 1 – 72 000 rubles;
- Class 2a – 104 000 rubles;
- Class 2b – 136 000 rubles;
- Class 3 – 184 000 rubles.
Cost calculation:
List of information required to calculate the cost of registering a medical device:
- Name of the medical device;
- Purpose and scope;
- Specifications;
- The materials from which the medical device is made;
- Analogues registered in the Russian Federation.
If you are interested in this service, please contact us
SERVICES
Fill out the form for feedback
By clicking on the button, I agree to the processing of personal data.